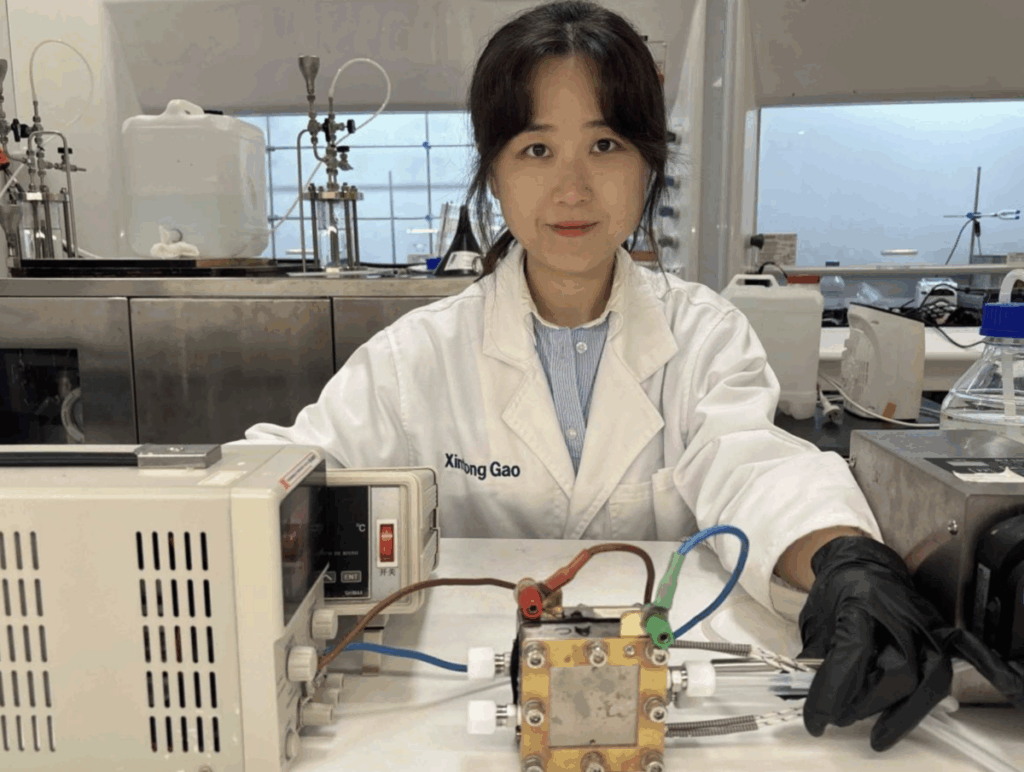
Australian researchers have developed electrolysis systems that use urine and waste water from urine and waste water to generate hydrogen against “considerably lower” energy costs than traditional methods for water splitting.
Researchers from the University of Adelaide And the Australian Research Council of Excellence for Carbon Science and Innovation (COE-CSI) have created two electrolysis systems that use urine in urine and waste water to produce hydrogen, while electricity consumption is reduced by a maximum of 27% compared to hydrogen production on water.
COE-CSI Chief Investigator Professor Yao Zheng said that the approach not only makes green hydrogen economically competing with fossil fuels derived alternatives, but also creates an elegant solution for waste water treatment.
“Although we have not solved all the problems, if these systems are scaled up, our systems produce harmless nitrogen gas instead of the toxic nitrates and nitrites, and both systems will use between 20-27% less electricity than water-splitting systems,” he said.
Hydrogen is usually generated by the use of electrolysis to split water into oxygen and hydrogen. The research team said that this process is “costs priceless” and energy intensive, where 1.23 V is needed to activate the reaction while they could split urea, which as a hydrogen carrier has a lower thermodynamic decay barrier than that of water, with only 0.37 V.
In their first study, the team used a membrane -free electrolysis system powered by a copper -based catalyst with pure urea as a raw material. That urea was produced by the Haber-Bosch ammonia synthesis process. To prevent this energy-intensive and carbon emittering process, a second system has been designed to use urea in urine.
“We have to reduce the costs for making hydrogen, but in a carbon neutral way,” said Zheng. “The system in our first paper, while the use of a unique membrane-free system and new copper-based catalyst, used pure urea, which is produced via the Haber-Bosch ammonia synthesis process that is energy-intensive and releases a lot of CO₂.”
“We have solved this by using a green source of urine, human urine, which forms the basis of the system investigated in our second paper.”
Although a promising source of urea, urine entails its own challenge. It contains chloride ions that can cause unwanted chemical reactions during electrolysis. These reactions produce chlorine gas that the anode of the system corrodes and undermines the long -term functionality.
To tackle this, the second system of the researchers has a chlorine-mediated oxidation mechanism that redirects the reaction route with the help of platinum-based catalysts supported on carbon.
The researchers said the approach not only protects the anode, but also maintains efficient hydrogen production from urine, adding the electricity consumption achieved on platinum-based system as 4.05 kWh per cubic meter of hydrogen, which performs better than traditional water electrolysis (4.70-5.00 kWh).
“The financial implications of this breakthrough could transform the green hydrogen landscape,” they said, adding that calculations suggest that hydrogen produced by their system costs less than traditionally “gray” hydrogen from fossil fuels.
“The membrane -free electrolyzer on an industrial scale based on this system successfully lowers hydrogen production costs To USD 1.81 per kilogram ($ 2.79), lower than that of gray hydrogen, and under the technical goal of the US Energy Energy from USD 2.00-2.50 ($ 3.08- $ 3.85) per kilogram, ”the researchers said.
The team is now working on building full membrane -free systems that restore both green hydrogen and restore nitrogen -rich waste water. It also recognized that the use of platinum as a catalytic material is untenable and wants to develop non-Precious metal alternatives.
The research has been published everywhere Angewandte Chemie International Edition And Nature communication.
This content is protected by copyright and may not be reused. If you want to work with us and reuse part of our content, please contact: editors@pv-magazine.com.